Top Ten Cancers in the Greater Bay Area: Incidence and Mortality,1988-2021
In our 2024 report, the Greater Bay Area Cancer Registry highlights the top ten cancers in the nine Bay Area counties of its region. We provide an overview of cancer incidence and mortality in the Greater Bay Area, and downloadable data sheets for each of the top ten cancer sites. For interactive graphics showing incidence and mortality for the most common cancers, please use the GBACR Dashboard.
Introduction
The Greater Bay Area Cancer Registry (GBACR), part of the California Cancer Registry (CCR) and the SEER program, is operated by the University of California, San Francisco (UCSF) and collects information on all newly diagnosed cancers occurring in residents of nine Greater Bay Area counties: Alameda, Contra Costa, Marin, Monterey, San Benito, San Francisco, San Mateo, Santa Clara, and Santa Cruz. Statewide cancer reporting began in California in 1988. At present, the most recent year of complete cancer ascertainment and follow-up for deaths is 2021.
This report highlights current cancer statistics for the ten most common invasive cancers, defined as tumors that have spread beyond the tissue of origin to other parts of the body. The report is based on available data for new cases of cancer and cancer deaths for the 34-year period from 1988 through 2021. Site-specific data sheets include incidence and mortality rates and information on trends in incidence and mortality over time and highlights the most recent five years of data from 2017-2021. Data were suppressed when less than 16 cancer cases were recorded in any given year or for populations under 10,000, for stability and reliability. As a result, information for males or females, or certain racial or ethnic groups may be missing, and between-group comparisons may not be reported. Statewide rates are included for comparison (see County-Specific Incidence and Mortality Rates worksheet and Comparison worksheet). A detailed guide to data in the files appears in the first worksheet entitled “README”.
Since 1988, the overall incidence and mortality rates of cancer (calculated as the number of new cases and deaths per 100,000 individuals in the population at risk, respectively) have greatly decreased in the Greater Bay Area. For each cancer site, there are notable differences in rates by sex, race, and ethnicity, but overall, there are promising patterns of decreasing incidence and mortality for most cancer sites. In the provided data files, we focus on sex- and racial and ethnic-specific cancer rates and trends for racial and ethnic groups including the Asian American, Native Hawaiian, and Pacific Islander (AANHPI), Hispanic, non-Hispanic (NH) Black and NH White populations. Population estimate data for calculating age-adjusted incidence rates for specific AANHPI ethnicities as well as other distinct racial and ethnic groups are needed for cancer surveillance in our diverse communities; yet these data are not available from the Census for recent years, including 2021. For highly heterogenous population groups, like AANHPIs, current limitations of available population estimates preclude systematic surveillance of trends in cancer rate trends for specific ethnic groups.
Tools
In addition to this report, there are several interactive tools available to further explore cancer statistics:
- GBACR Dashboard which provides both cancer incidence and mortality rates by county, race, ethnicity, sex, age, and year of diagnosis.
- California Health Maps website allows users to access incidence rates for several geographies, including census tract aggregation zones, medical service study areas, census designated places, and legislative districts.
- Cal*Explorer is an interactive website that provides easy access to a wide range of CCR cancer statistics. It provides detailed statistics for cancer sites by gender, race, age, region, and for a selected number of cancer sites, by histology.
Glossary of Technical Terms
Analytic Terms
Incidence: The number of new cases of cancer diagnosed in a certain period of time. In this report, incidence data are based on the number of new cases of cancer diagnosed in residents of the Greater Bay Area over the period January 1, 1988 through December 31, 2020.
Mortality: The number of deaths due to cancer in a certain period of time. In this report, mortality data are based on the number of deaths from cancer in residents of the Greater Bay Area over the period January 1, 1988 through December 31, 2020.
Incidence/mortality rate: The number of new cancer cases (incidence) or deaths (mortality) in a certain period of time in a specific population, divided by the size of that population. Incidence and mortality rates are expressed per 100,000 population. In this report, annual and cumulative (or average) 5-year incidence and mortality rates are presented.
Confidence interval: A statistical measure of the precision of the observed incidence or mortality rate. The observed rate is an estimate of the true rate based on counts of cancer cases (or deaths) and of the population, and is subject to variation from the true value of the rate. The confidence interval for the observed rate is a range of values within which the true rate is thought to lie, with a specified level of confidence, e.g., 95%. Rates based on larger numbers of cases (or deaths) are subject to less variation.
Age-adjusted incidence/mortality rate: Age-adjustment is a statistical method that allows comparisons of incidence and mortality to be made between populations with different age distributions. An age-adjusted cancer incidence (or mortality) rate is defined as the number of new cancers (or deaths) per 100,000 population that would occur in a certain period of time if that population had a ‘standard’ age distribution. In this report, incidence and mortality rates are age-adjusted using the U.S. 2000 Standard Population.
Trend: Used to describe the change in the incidence or mortality rate over time. The Annual Percent Change (APC) is used to measure trends. For example, incidence rates may rise gradually over a period of several years, then drop sharply for several years. Statistical criteria are used to quantify the magnitude of change over a period of time.
Race and ethnicity: In this report, race and ethnicity are categorized as: All races and ethnicities, Asian American/Pacific Islander (AAPI), Hispanic, non-Hispanic (NH) Black, or NH White. “All races and ethnicities” category includes the four categories listed plus American Indian/Alaska Native (AIAN) people and people with another or unknown race and ethnicity. The latter two groups are not consistently reported separately due to small numbers for many cancer sites (<15 cases).
Cancer Terms
Carcinoma: Cancer that begins in the skin or in tissues that line or cover internal organs.
Histology: The study of tissues and cells under a microscope. Cancers are identified and diagnosed primarily on the basis of histology. They often are classified further by histologic subtype.
In situ: Meaning ‘in its original place’. For example, in carcinoma in situ, abnormal cells are found only in the place where they first formed and have not spread.
Invasive: Cancer that has spread beyond the layer of tissue in which it developed and is growing into surrounding, healthy tissues. Also called infiltrating cancer. Invasive tumors are classified according to how far the cancer has spread at the time of diagnosis.
Malignant: Cancerous cells that can invade and destroy nearby tissue and spread to other parts of the body.
Stage: The extent of the cancer in the body, such as how large the tumor is, and if it has spread. In this report, four categories of stage are used: (1) In situ (see above), (2) localized – cancer is limited to the place where it started with no sign that it has spread, (3) regional – cancer has spread to nearby lymph nodes, tissues or organs, (4) distant – cancer has spread to distant parts of the body.
Risk Factor: A characteristic that is associated with the disease; a variable that may increase the chances of getting a specific type of cancer.
Population data used for calculating rates
Beginning with this data release, we use the 2010-2019 intercensal population estimates that were produced by Woods & Poole Economics, Inc. through a contract with the NCI SEER program. The methodology follows previously published Census methodologies to align closely with the anticipated Census Bureau’s intercensal data that are tentatively scheduled to be released in late 2024. Read more about population estimates for this data release here.
Incidence and Mortality in the Greater Bay Area, 1988-2021
Rates of invasive cancers have decreased significantly during the 34-year period from 1988 through 2021 in the Greater Bay Area. Significant declines were also noted by the American Cancer Society in their Annual Cancer Statistics report [1].
Last year, we reported that we observed 9.6% and 9.3% fewer cancer cases diagnosed among males and females in 2020, respectively, compared to expected counts calculated from previous years. These estimates were impacted by the COVID-19 pandemic and the limited access to cancer-related healthcare services throughout the United States. Starting in 2023, SEER recommended excluding the 2020 incidence data from the estimation of trends (see Impact of COVID on the April 2023 SEER Data Release). As a result, trends for incidence exclude the 2020 data point (rate). The 2021 cancer case counts are aligned with expectations prior to 2020.
The five most common invasive cancers—breast, prostate, lung and bronchus, colorectal, and uterine—accounted for slightly over half of all newly diagnosed cancers in the Greater Bay Area. Lung and bronchus, breast, prostate, colorectal, and pancreatic cancers were the most common cause of cancer deaths, collectively accounting for half of all cancer deaths in the Greater Bay Area.
Incidence and Mortality Trends Over Time
- Since 1988, yearly incidence of invasive cancer has declined more among males (-0.9%) than females (-0.2%). This is driven largely by declines in the incidence of smoking-related cancers (e.g., lung and bronchus) and prostate cancer in males. In more recent years, since 2013, incidence among males and females has been stable (excluding the 2020 incidence rate for calculation of recent trends).
- As with incidence, a significant annual decline has occurred for mortality since 1988 (males: -2.0%; females: -1.7%). In more recent years, from 2011 through 2021, males and females combined cancer mortality declined by -2.3% per year. Trends were comparable when looking at males and females separately.
Incidence, 2017-2021
- In 2021, 35,298 invasive cancers were diagnosed in the Greater Bay Area.
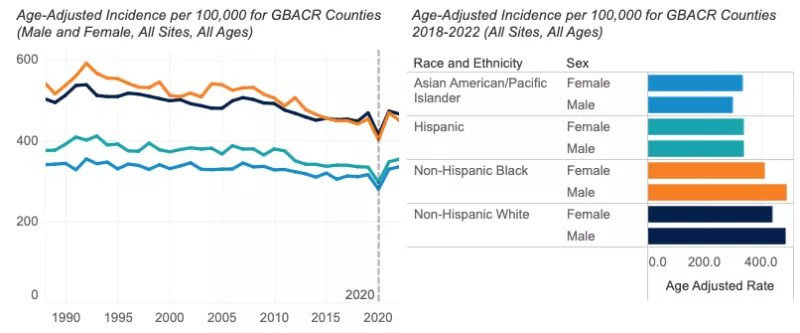
- The incidence of all invasive cancers from 2017-2021 was higher in males than females.
- Males: incidence was highest among NH Black males (466.6 per 100,000), followed by NH White (464.2 per 100,000), Hispanic (340.2 per 100,000), and AANHPI males (283.1 per 100,000).
- Females: NH White females had the highest cancer incidence (425.4 per 100,000), followed by NH Black (388.9 per 100,000), Hispanic (327.7 per 100,000), and AANHPI (312.8 per 100,000) females.
- Among Greater Bay Area counties, Santa Cruz County had the highest overall cancer incidence among males and females combined (457.0 per 100,000), followed by Marin County (437.6 per 100,000), driven by higher incidence of female breast cancer and melanoma, particularly among males.
- Cancer incidence among males was significantly lower in the Greater Bay Area than California overall (405.5 vs. 414.2 per 100,000). Among females, overall cancer incidence was similar between the Greater Bay Area and all of California (379.0 vs. 380.8 per 100,000, respectively).

Mortality, 2017-2021
- In 2021, 10,421 cancer deaths occurred in the Greater Bay Area.
- Cancer mortality from 2017-2021 was higher in males than females.
- Males: mortality was highest among NH Black males (199.1 per 100,000), followed by NH White (145.5 per 100,000), Hispanic (126.5 per 100,000), and AANHPI males (110.7 per 100,000).
- Females: NH Black females had the highest cancer mortality (146.8 per 100,000), followed by NH White (111.6 per 100,000), Hispanic (97.4 per 100,000), and AANHPI (83.3 per 100,000) females.
- From 2017 through 2021, overall cancer mortality in the Greater Bay Area was significantly lower than California for both males (138.2 vs. 157.4 per 100,000) and females (105.2 vs. 117.6 per 100,000)
Data Tables - All Cancer Sites
References
[1] Siegel, R. L., K. D. Miller, N. S. Wagle, and A. Jemal. "Cancer Statistics, 2024." CA Cancer J Clin 74, no. 1 (Jan 2024): 12-49. https://doi.org/10.3322/caac.21820
Female Invasive Breast Cancer
Invasive breast cancer is the most common cancer in females, accounting for approximately a third of all invasive cancers diagnosed annually in the Greater Bay Area and in California. About one in eight females in the United States (U.S.) will develop invasive breast cancer within their lifetime. Risk factors include older age, family history of breast cancer, inherited genetic mutations (BRCA1 and BRCA2), early age of menarche, late age of menopause, no pregnancies or pregnancies later in life (i.e., first pregnancy after age 30), postmenopausal hormone therapy use, obesity and excessive weight gain, physical inactivity, alcohol consumption, and dense breast tissue (as indicated on a mammogram). However, risk factors differ across subtypes of breast cancer [1-4]. The U.S. Preventive Services Task Force recommends biennial screening mammography for breast cancer for most females 40 to 74 years of age. This recommendation states that current evidence is insufficient to assess the balance of benefits and harms of screening mammography in females more than 75 years of age [5].
References
[1] National Cancer Institute. SEER Cancer Stat Facts: Female Breast Cancer. National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/statfacts/html/breast.html
[2] Song, M. and E. Giovannucci, Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncol, 2016. 2(9): p. 1154-61.
[3] Sprague, B.L., et al., Proportion of invasive breast cancer attributable to risk factors modifiable after menopause. Am J Epidemiol, 2008. 168(4): p. 404-11.
[4] Tamimi, R.M., et al., Population Attributable Risk of Modifiable and Nonmodifiable Breast Cancer Risk Factors in Postmenopausal Breast Cancer. Am J Epidemiol, 2016. 184(12): p. 884-893.
[5] Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. JAMA, 2024. 331(22): p. 1918-1930. Available at: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening.
Prostate Cancer
Prostate cancer was the most commonly diagnosed cancer among Greater Bay Area males in the years 1988 through 2021. Prostate cancer typically develops slowly, and males diagnosed with non-metastatic prostate cancer are more likely to die with the disease than of it. Risk factors include family history and increasing age. Prostate cancer incidence rates in the U.S. spiked in 1992 then steadily declined, a trend that has been attributed to the widespread adoption of PSA testing. [2] However, the incidence has increased by 2.9% per year from 2014 through 2021, with the greatest increase in incidence among people diagnosed with advanced stage disease. The lifetime risk of being diagnosed with prostate cancer is approximately 11.0% and risk of dying from prostate cancer is 2.5% [3]. Currently, the U.S. Preventive Services Task Force recommends that males 70 years and older should not be screened for prostate cancer and those aged 55-69 should discuss with their clinician the relative risks and benefits of early detection and treatment, and then make an individual decision whether to be screened [3].
References
[1] PDQ® Screening and Prevention Editorial Board. PDQ Prostate Cancer Screening. Bethesda, MD: National Cancer Institute. Updated 05/06/2022. Available at: https://www.cancer.gov/types/prostate/patient/prostate-screening-pdq. [PMID: 26389306]
[2] Siegel, R., et al., Cancer Statistics, 2023. CA Cancer Journal, 2023. 73(1): p.17-48. Available at: https://acsjournals.onlinelibrary.wiley.com/doi/pdf/10.3322/caac.21763
[3] US Preventive Services Task Force. Grossman, S. J. Curry, D. K. Owens, K. Bibbins-Domingo, A. B. Caughey, K. W. Davidson, C. A. Doubeni, M. Ebell, J. W. Epling, Jr., A. R. Kemper, A. H. Krist, M. Kubik, C. S. Landefeld, C. M. Mangione, M. Silverstein, M. A. Simon, A. L. Siu, and C. W. Tseng. "Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement." JAMA 319, no. 18 (May 8 2018): 1901-13. https://dx.doi.org/10.1001/jama.2018.3710.
Lung and Bronchus Cancer
Smoking remains by far the leading risk factor for lung and bronchus cancer (hereafter lung cancer) [1]. In California, the prevalence of tobacco smoking continues to decline; in 1988, 23.7% of Californians smoked compared to 6.2% in 2021 [2]. However, despite these declines, lung cancer remains the second-most common cancer among males (behind prostate) and females (behind breast) and the most common cause of cancer deaths in the Greater Bay Area, California, and nationwide [3].
References
[1] Lung Cancer Prevention (PDQ): https://www.cancer.gov/types/lung/patient/lung-prevention-pdq
[2] California Department of Public Health, California Tobacco Control Program. California Tobacco Facts and Figures 2022. Sacramento, CA: California Department of Public Health; May 2023.
[3] American Cancer Society. Cancer Facts & Figures 2024. Atlanta: American Cancer Society; 2024.
Colorectal Cancer
Colorectal cancer was the fourth most common cancer diagnosed in the Greater Bay Area in the most recent years (2017-2021). Usually, these cancers develop when tissue in the inner surface of the colon or rectum starts to grow, forming a polyp [1]. Older age, obesity, smoking, history of colorectal polyps, alcohol consumption, and a diet high in red and processed meats are associated with increased risk of this cancer [1-3]. Adhering to colorectal cancer screening guidelines, engaging in regular physical activity, and a diet rich in whole grains and dairy products are associated with lower risk of colorectal cancer [3]. Colorectal cancer screening is important because it can identify polyps that could lead to in situ or invasive cancer, allowing for early intervention (removal of the polyp). The U.S. Preventive Services Task Force recommends screening for colorectal cancer in adults aged 45-75 years [4]. While incidence of colorectal cancer is decreasing overall, the incidence of colorectal cancer is increasing among people less than 50 years of age throughout the U.S., including California [5,6].
Data Tables - Colorectal Cancer
References
[1] National Cancer Institute, SEER Cancer Statistics Factsheets: Colon and Rectum. Available at: http://seer.cancer.gov/statfacts/html/colorect.html. U.S. Department of Health and Human Services, National Cancer Institute, Bethesda, MD.
[2] National Cancer Institute, Colon Cancer Treatment-Patient Version (PDQ). Available at: https://www.cancer.gov/types/colorectal/patient/colon-treatment-pdq#section/_112. U.S. Department of Health and Human Services, National Cancer Institute, Bethesda, MD.
[3] World Cancer Research Fund International, Colorectal Cancer. Available at: https://www.wcrf.org/diet-activity-and-cancer/cancer-types/colorectal-cancer/. Accessed on June 28, 2023. WCRF International, London.
[4] US Preventive Services Task Force Recommendation Statement. Screening for Colorectal Cancer: JAMA, 2021. 325(19): p. 1965-1977.
[5] Ellis, L., et al., Colorectal Cancer Incidence Trends by Age, Stage, and Racial/Ethnic Group in California, 1990-2014. Cancer Epidemiol Biomarkers Prev, 2018.
Uterine Cancer
Uterine cancer is the most common gynecologic cancer and is primarily diagnosed in post-menopausal females, with incidence peaking in the sixth decade of life [1]. In addition to age, other risk factors include obesity, estrogen-only hormone replacement therapy, and family history of uterine, colon, or ovarian cancer. Endometrial cancer (lining of the uterus) accounts for more than 90% of uterine cancers [1,2]. Rates and trends should be interpreted carefully due to the difficulty in identifying true at-risk populations (females who have not had a hysterectomy), which may vary across time and racial and ethnic groups. For example, NH Black females experience higher hysterectomy rates in the U.S., partly due to higher prevalence of uterine fibroids. [3] However, hysterectomy data is not available and thus not used in these calculations.
References
[1] Centers for Disease Control and Prevention. What Are the Risk Factors for Uterine Cancer?; 2024 Feb 23. Available from: https://www.cdc.gov/uterine-cancer/risk-factors/index.html
[2] Cancer.Net. Uterine Cancer - Statistics; 2024 Jan 17. Available from: https://www.cancer.net/cancer-types/uterine-cancer/statistics
[3] Temkin, Sarah M et al. “The End of the Hysterectomy Epidemic and Endometrial Cancer Incidence: What Are the Unintended Consequences of Declining Hysterectomy Rates?.” Frontiers in oncology vol. 6 89. 14 Apr. 2016, doi:10.3389/fonc.2016.00089
Invasive Melanoma
Invasive melanoma, a cancer of the skin’s pigment cells, is substantially more common among populations with fair complexions and prolonged exposure to ultraviolet (UV) light from the sun or tanning beds. Melanoma is not exclusive to NH White persons, however. It is substantially more common among NH White males than NH White females. In the Greater Bay Area, among NH White males, melanoma was the second most common newly diagnosed invasive cancer. Compared to other types of skin cancers, melanoma is more likely to spread to other parts of the body [1].
References
[1] National Cancer Institute, Melanoma Treatment (PDQ®)–Patient Version. Available at: https://www.cancer.gov/types/skin/patient/melanoma-treatment-pdq. U.S. Department of Health and Human Services, National Cancer Institute, Bethesda, MD.
Non-Hodgkin Lymphoma
Non-Hodgkin Lymphoma is a cancer that starts in cells called lymphocytes, which are part of the body’s immune system. Lymphomas can start anywhere that lymph tissue is found, such as lymph nodes, the spleen, bone marrow, and the tonsils [1]. Lymphomas can be indolent, meaning the cancer does not need immediate treatment but should be monitored closely. They can also be aggressive, requiring immediate treatment due to their ability to grow and spread quickly. Factors affecting an individual’s risk of developing Non-Hodgkin Lymphoma include immune disorders, infections, genetics, family history, and occupational factors [2,3].
Data Tables - Non-Hodgkin Lymphoma
References
[1] American Cancer Society, Non-Hodgkin Lymphoma. 2024 Feb 15: Available at: https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/what-is-non-hodgkin-lymphoma.html
[2] SEER Cancer Stat Facts: Non-Hodgkin Lymphoma. National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/statfacts/html/nhl.html
[3] Armitage, J.O., et al., Non-Hodgkin lymphoma. Lancet, 2017. 390(10091): p. 298-310.
Bladder Cancer
Bladder cancer is the eighth most common cancer and four times more prevalent among males than females. Approximately 90% of cases occur in those ages 55 and over. NH White males and females are more likely to be diagnosed with bladder cancer than any other racial and ethnic group. The largest risk factor for bladder cancer is smoking tobacco, which contributes to 50-65% of all cases; up to another 20% of bladder cancer can be attributed to exposure to chemicals in textile, rubber, leather, and print industries [1].
References
[1] Saginala, K., Barsouk, A., Aluru, J. S., Rawla, P., Padala, S. A., & Barsouk, A. (2020). Epidemiology of Bladder Cancer. Medical Sciences, 8(1), 15. https://doi.org/10.3390/medsci8010015
Kidney Cancer
Kidney cancer is one of the top 10 most common cancers in the U.S. and is about twice as common in males than females [1]. Established risk factors for kidney cancer include tobacco smoking, obesity, and history of hypertension and chronic kidney disease [2]. Most kidney cancers (between 60-70%) are diagnosed before cancer has spread outside the kidney (localized stage), and the observed incidence trends are driven by the trends in localized disease [3,4]. The increasing incidence rate can in part be attributed to the greater use of medical imaging procedures, which results in incidental detection of early kidney cancers. Increasing kidney cancer incidence may also reflect changes in the prevalence of kidney cancer risk factors, such as obesity and hypertension, in the population [4].
References
[1] American Cancer Society, Kidney Cancer: 2024 May 1: Available at: https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html
[2] Scelo G, Larose TL. Epidemiology and Risk Factors for Kidney Cancer. J Clin Oncol. 2018 Oct 29;36(36):JCO2018791905. doi: 10.1200/JCO.2018.79.1905.
[3] Kase AM, George DJ, Ramalingam S. Clear Cell Renal Cell Carcinoma: From Biology to Treatment. Cancers (Basel). 2023 Jan 21;15(3):665. doi: 10.3390/cancers15030665.
[4] Rossi, S. H., Klatte, T., Usher-Smith, J., & Stewart, G. D. (2018). Epidemiology and screening for renal cancer. World journal of urology, 36(9), 1341-1353.
Pancreatic Cancer
Pancreatic cancer is the 10th most common cancer and the 5th most common cause of cancer mortality, in the Greater Bay Area. Pancreatic cancer is often detected at a later stage due to its rapid spread and the lack of symptoms in the early stages. Later stages are associated with symptoms, but these can be non-specific, such as lack of appetite and weight loss [1]. Smoking, obesity, personal or family history of diabetes or pancreatitis, occupational exposure to some chemicals, and certain hereditary conditions have been associated with risk of pancreatic cancer. Pancreatic adenocarcinoma is the most common type of pancreatic cancer, accounting for approximately 85% of pancreatic cancers. [2]
Data Tables - Pancreatic Cancer
References
[1] National Cancer Institute, SEER Cancer Statistics Fact Sheets: Pancreatic Cancer. Available at: http://seer.cancer.gov/statfacts/html/pancreas.html. U.S. Department of Health and Human Services, National Cancer Institute, Bethesda, MD.
[2] Ilic, M. and I. Ilic, Epidemiology of pancreatic cancer. World J Gastroenterol, 2016. 22(44): p. 9694-9705.